a) Large Single Crystal Growth of Diamond
b) Bulk growth of Gallium Nitride
Gallium nitride is a direct bandgap (3.45eV) semiconductor that has the potential to revolutionize the fields of solid state lighting, electronics and power conversion. GaN's inherent high breakdown strength, high saturation electron velocity and good thermal conductivity make this material very promising for power switches applications; helping to reduce the consumption of energy in grid, automotive and industrial applications.
Despite the huge technological interest, the progress in high power electronics and long-term applications has been slow due to the high cost of low defect density bulk GaN wafers. GaN crystals cannot be grown using typical Bridgman or Czochralski type techniques because decomposition occurs prior to melting. Vapor phase heteroepitaxy on single crystal substrates such as SiC, and sapphire has been the most common method to synthesize GaN. However, the large threading dislocation density (~ 1010 - 1014 cm-2) obtained in heteroepitaxial growth due to lattice mismatch between the GaN and the underlying substrate 2 is significantly higher than the required
for high power devices (<104 cm-2). Bulk synthesis methods have been used to obtain GaN crystals with low defect density. Among these, the ammono-thermal method has been the one that provides the best quality.3 In general, bulk synthesis methods are performed under extreme temperatures and pressures for long periods of time (in the order of days) making them costly prohibited. The high costs of producing GaN based power electronics makes this material noncompetitive and limits its widespread use.
Our group at Conn Center has been working to produce at low cost large area (~ 1-2 inch) single crystal GaN wafers with low defect density (<107 cm2). In contrast to other bulk growth techniques, our proposed processes are performed at low pressures (sub-atmospheric), relatively low temperatures (~900 C) and are considerably faster (in the order of hours) making them easy to scale and more importantly, cheaper than current GaN bulk techniques. If successful, these techniques will enable wide spread applications of GaN in power electronics and eventually could result in enormous energy savings.
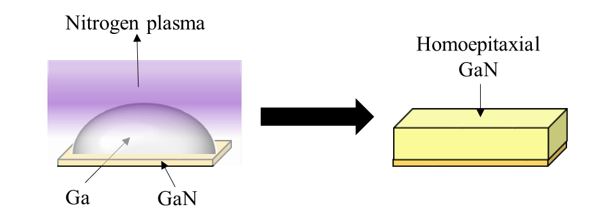
Homoepitaxial growth of GaN via plasma-assisted liquid phase epitaxy (LPE). GaN is grown homoeptaxially by a controlled nitridation of Ga using nitrogen plasma. This technique offers a contaminant-free and safe environment as it only uses Ga and N2 as the precursors instead of TMG and NH3 in the case of MOCVD, or HCL, Ga, and NH3 in the case of HVPE.
c) Halide Vapor Phase Epitaxy(HVPE) of Novel III-V Materials
Conn Center researchers have identified a number III-V alloys whose band gaps and band edge energetics can be engineered for various solar cell and solar fuel applications. However, their deposition/synthesis methods are either complicated or expensive. Here, Conn Center researchers are developing a halide/hydride vapor phase approaches for developing high growth rate methods for growing near single crystal quality films. The long term objectives include the development of roll to roll processing of these materials systems for large area growth and flexible substrates.
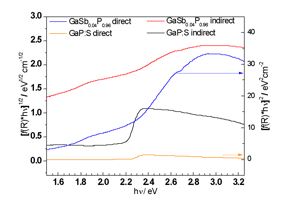
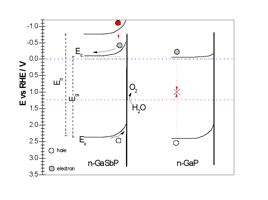
Left, Tauc plots of diffuse reflectance measurements of a GaSbP alloy containing 4 % of Antimony, compared to pure GaP. Right, proposed band edge alignments of the ternary alloy versus pure GaP.
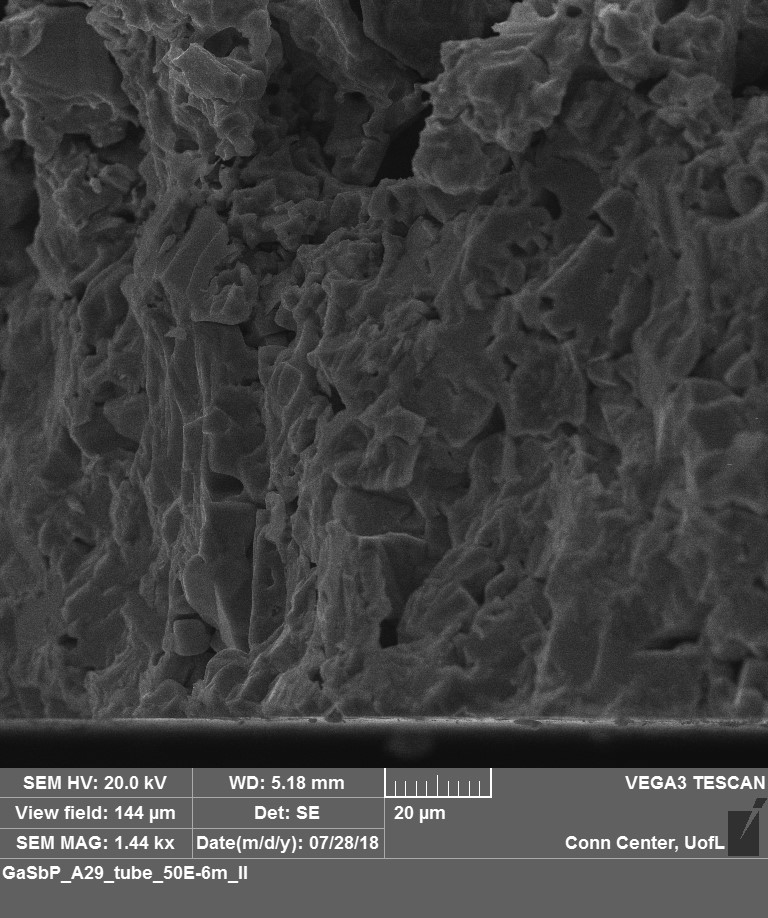
Scanning Electron Micrograph of the GaSbP alloy cross section.
d)Metal Oxide Nanowires and Complex Metal Oxide Nanoparticles
Metal oxide nanowires and complex metal oxides play an important role in various energy conversion and storage applications. Examples include heterogeneous catalysts and electrocatalysts. Advanced Energy Materials, LLC, a company started with original efforts from Conn Center, has been pioneering the scalable manufacturing of both metal oxide nanowires and complex metal oxide particles with precise compositional control.
Nanowires provide uniformity of active surfaces, great stability and improved diffusion processes among other advantages, that's why they have attracted a lot of attention into material design in recent years. In our group the development of solid adsorbents in nanowire morphology have been studied successfully with major focus in carbon capture technologies where the nanowire morphology enhanced the adsorption kinetics and provide stability for the recycling of adsorbent ceramics.5 Materials synthesized through solvo-plasma or thermal oxidation techniques have described ultrafast CO2 uptake reaching surface saturation in 3 minutes when typically this region is reached after 80 minutes.
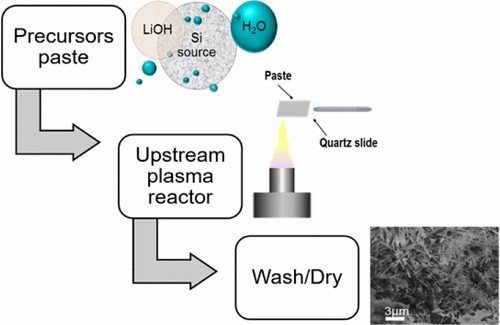
Figure 3. A schematic illustration of various steps used in our solvo-plasma synthesis method.
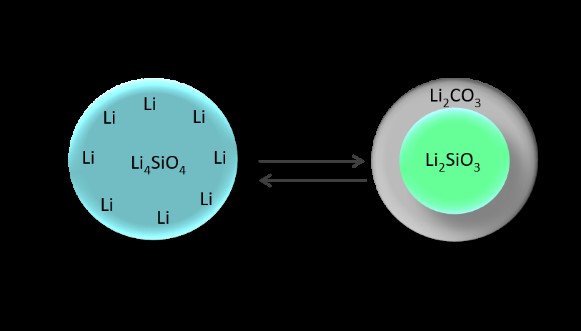
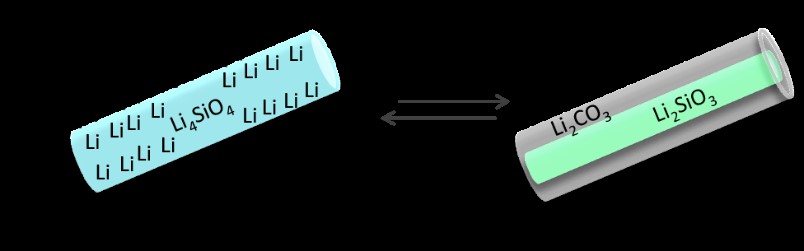
Figure 4. Mechanistics of the morphology enhanced CO2 capture and Full isotherms of CO2 adsorption (60%CO2/N2) on Li silicates: (a) agglomerates between 10 and 200 μm, (b) 2-20 μm particles, and (c) nanowire aggregates, with diameters less than 50 nm and lengths around 5 μm. Schematic.
Electrochemical conversion of CO2 into energy-dense liquids, such as formic acid, is desirable as a hydrogen carrier and a chemical feedstock. SnOx is one of the few catalysts that reduce CO2 into formic acid with high selectivity but at high overpotential and low current density. We show that an electrochemically reduced SnO2 porous nanowire catalyst (Sn-pNWs) with a high density of grain boundaries (GBs) exhibits an energy conversion efficiency of CO2-into-HCOOH higher than analogous catalysts. HCOOH formation begins at lower overpotential (350 mV) and reaches a steady Faradaic efficiency of ca. 80% at only -0.8 V vs. RHE. A comparison with commercial SnO2 nanoparticles confirms that the improved CO2 reduction performance of Sn-pNWs is due to the density of GBs within the porous structure, which introduce new catalytically active sites. Produced with a scalable plasma synthesis technology, the catalysts have potential for application in the CO2 conversion industry[7].
e) Novel Processing Using Plasma Catalysis
Conn Center researchers are working to develop fundamental understanding of catalytic phenomena and exploit plasma catalysis to advance catalyst synthesis, design and their synergistic interactions, enabling transformative advances in chemical transformation of nitrogen, methane and carbon dioxide.
Grand Challenges in Chemical Transformations: The chemical transformation of nitrogen (N2), methane (CH4) and carbon dioxide (CO2) into technologically relevant chemicals is a grand challenge in catalysis science, with a potentially enormous impact on our energy infrastructure. Specifically, these three species are relatively inert, and the key rate-limiting step in their chemical transformation is the dissociation of these molecules. The energetic barrier of dissociation can be decreased effectively using synergistic action between gas phase activation combined with catalysis.
The most important transformation routes for the above three molecules are the following:
(a) Conversion of nitrogen and hydrogen to ammonia for fertilizers and fuel; (b) conversion of methane to higher fuels and plastics, and (c) CO2 conversion, dry reforming and fuel production. The overall reactions are shown below.
Ammonia Synthesis: N2 + 3H2 ⇔ 2NH3 (Haber-Bosch)
Methane Processing: 2CH4 + O2 ⇔ C2H4 + 2H2O (Oxidative coupling of methane)
CO2 Reduction: CO2 + CH4 ⇔ 2CO + 2H2 (Dry reforming of methane)
CO2 + 2H2O ⇔ CH3OH + 3/2 O2 (Artificial photosynthesis)
Current catalysts and processes typically have low conversion, high temperature and pressure process conditions, short life span, and more often than not, severe environmental impacts.
Proposed Concept of Plasma Catalysis: The concept of plasma catalysis has grown in significance in the last ten years or so.7 Plasma can be created via exciting gas phase molecules with an applied electric field through ionization and electron-molecule reactions. The energized neutral or charged species can directly impact the catalytic process by significantly decreasing the energetic barrier, or by changing the reaction pathway, eventually enhancing the rate of reaction. In conventional catalysis, the gas phase molecules (reactants) are exposed to catalyst beds at high temperatures and pressures to overcome the energetic barrier, resulting in dissociation of gas phase molecules. The dissociated species further react to form desired products. Second, the activity and selectivity depend on the nature of
catalyst materials in terms of composition, surfaces, and defects, as well as on processing conditions. Third,
plasmas present as non-equilibrium techniques for synthesizing complex materials and at time scales necessary for both discovery and development of new catalyst materials in terms of scalability and throughput.