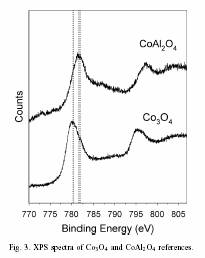
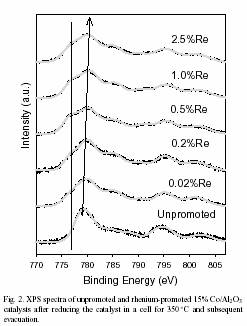
The addition of Re metal to Co/Al 2O 3 catalysts increases both the efficiency and extent of reduction of the cobalt oxide to active cobalt metal. This occurs by a two-step process, first the reduction of Co 3O 4 to CoO followed by further reduction to Co metal. The formation of Co metal is seen as a new shoulder at 777 eV in the Co 2p3/2 XPS region. With higher loadings of Re metal, there is a greater propensity to form a cobalt species with a 2p3/2 peak at a binding energy of 780 eV or slightly higher. This new cobalt containing species is identified as cobalt aluminate (CoAl 2O 4) based on the higher binding energy position and comparison to known reference standards.