

We recently reported a synthesis technique that paved the way for a new class of carbon tubular morphologies: ‘Carbon Microtubes’. As the name implies the internal diameters of the carbon tubes synthesized are in the range of microns. The distinct feature of this technique is that we can precisely control the morphology (shape) of structure in-situ during growth. Such control over the morphology cannot be attained using conventional carbon nanotubes (CNT) synthesis routes that use solid metal catalyst.
In our method we use low melting liquids such as gallium and indium to assist the growth of carbon tube. These low melting metals have very high surface energies and do not normally wet carbon. We use gas phase chemistry variations to change the wetting behavior of molten metals such as gallium. Specifically, the contact angle between gallium and carbon can be reduced by introducing gas phase impurities such as oxygen and nitrogen. Using variations in the gas phase chemistries we were able to synthesize various morphologies (shapes). Some of them are: Cones, straight tubes, Funnels, Tube-on-cone, n-staged morphologies and Y-junctions.
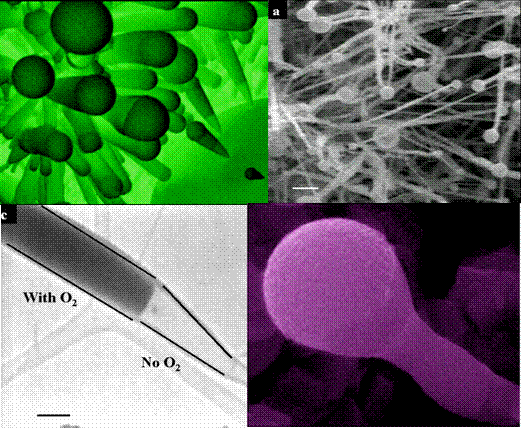
Figure 1. Different morphologies synthesized by changing the gas phase chemistry during growth.
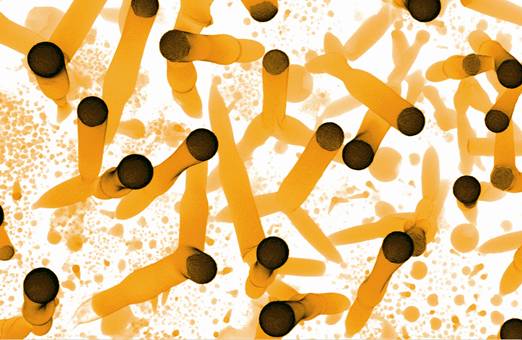 Figure 2. Seamless Y-junctions formed due to impingement of two individually growing carbon microtubes.
Figure 2. Seamless Y-junctions formed due to impingement of two individually growing carbon microtubes.
The morphologies shown above have never been synthesized earlier. This new breed of larger diameter carbon tubes with different morphologies have tremendous applications in Microfluidics as ‘Fluid flow & distribution channels’ and ‘microreactors’. Also due to their unique wall structure and larger diameters these could be used as ‘intercalation medium’ in lithium batteries. We are currently working on building microfluidic devices and are in the process of scaling up the process for large quantity production of carbon microtubes.
