Chemical Vapor Deposition of Diamond
The understanding of nucleation and growth of diamond is critical in making defect free, large size diamond crystals. The limited understanding of the effect of process conditions and impurities on the defect origin and the resulting morphology is the main driving force for this research.
Until now, the only way one can distinguish the process window is by looking at the feed gas composition. Recently, we developed a new phase diagram based on radical species that exist inside the reacting environment. Even though the amount of dopants may not influence the feed gas composition significantly but may influence the radical species composition. In addition, the dopant incorporation process affects the molecular level processes that take place during the growth , i.e., step propagation, step nucleation and defect formation. In addition, this new C-H-O ternary diagram based on radical species composition is finding applications in predicting diamond deposition inside trenches, onto complicated geometries and with the presence of gas phase impurities such as Sulfur.
In the experimental portion to determine the defect origin, we use microwave/hot-filament chemical vapor deposition reactors and scanning probe microscope to perform interrupted growth characterization experiments - pseudo in-situ growth characterization at nano-scale. Also, we complement these studies with kinetic MC simulations which are described in another section under KMC simulations.
1. C-H-O ternary diagram based on radical species composition
A process diagram for predicting diamond deposition was developed using steady state gas phase calculations. During diamond CVD process carbon can precipitate either non-diamond sooth or diamond or not deposit at all (no growth) depending on carbon supersaturation in the gas phase. Under very high supersaturations non-diamond deposits are obtained and under very low supersaturations there is no-growth. Only under a very narrow feed gas compositional window diamond growth occurs. These different deposition regimes are commonly known as ‘non-diamond domain’, ‘diamond domain’ and ‘no-growth domain’. Based on the feed gas compositions a C-H-O ternary diagram was created to predict diamond deposition domain (Figure 1a). However, this diagram cannot predict what happens inside the reactor, i.e., using radical species composition. This is important if one were to look at diamond deposition inside trenches, vias or onto complicated geometries or scale-up and also to look at effects that gas phase impurities may have on the deposition of diamond versus non-diamond phases.
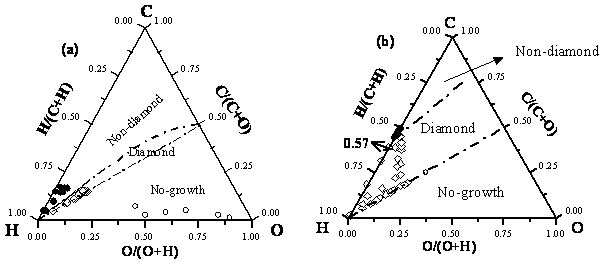
Figure 1. C-H-O ternary diagram (a) based on feed gas compostions, (b) based on steady state radical species composition.
The process diagram that we developed can predict what kind of deposition can happen if a particular set of process variables are chosen. These process variables could be temperature, pressure, feed gases and their compositions, kind of gas phase dopants and their compositions. To develop the process diagram steady state compositions were calculated for feed gas compositions chosen from all three diamond domains. All the species (C, H and O containing species) considered in the calculations are divided into ‘participating’ and non-participating species. The total atomic fractions of C, H and O from participating species were plotted onto a ternary diagram as shown in Figure 1. The positions of the data points fall into the 3 deposition domains.
The above ternary diagram in Fig. 1 should be able to predict deposition based on the radical species compositions inside the reactor rather than just the feed gas compositions. Any changes made to the system will change the species compositions and can change the deposition domain. For example, addition of sulfur to the feed gases has a drastic effect on the feed gas compositions.
The effect of sulfur on the diamond deposition domain
Using steady state calculations involving gas phase species containing C, H, O and S, we were able to predict the changes in deposition domain when sulfur is added to the feed gases. The gas phase carbon super-saturation is adjusted such that adding sulfur to feed gas compositions chosen from non-diamond and no-growth domain changes the domain to diamond domain. In the case of feed gas compositions chosen from diamond domain there is no change in the domain. In all the three cases, there is a decrease in CH 3 and C 2H 2 species with corresponding increase in CH 4. This could explain the observed decrease in growth rate with sulfur addition.
1.2 Predicting diamond deposition in high aspect ratio vias (trenches)
Deposition of diamond in high aspect ratio vias is a challenge due to the change in deposition domain along the depth of the vias. Using one-dimensional transport analysis and our process diagram we could successfully predict the variations in the deposition domain along the depth of vias with different aspect ratio.
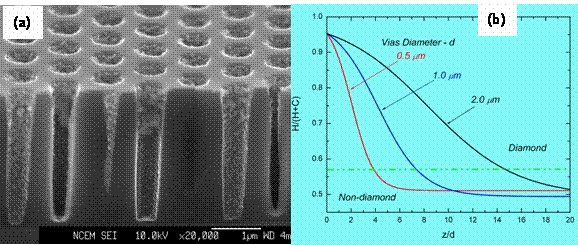
Figure 2: (a) High aspect ration silicon vias. (b) Diamond deposition vs. vias diameters
According to our calculations, the decrease in H concentrations is far greater than the decrease in CH 3 composition along the depth (Figure 3). This relatively high depletion of H causes graphite or non-diamond sooth to the stable phase. H is known to inhibit the formation and stabilization of graphite in diamond CVD. A higher decrease in H causes a transition from diamond domain at the mouth to non-diamond domain at some point along the depth, depending on the vias diameters. This variation is shown in Figure 2b. However, in order to increase the depth at which diamond can be formed, we could use O 2 in the feed gas. This use of O 2 in the feed gases introduces O and OH species that also act as inhibitors for graphite stabilization. Our analysis indicated that addition O 2 to CH 4/H 2 feed gases will increase the depth of diamond deposition. This is due to the fact that the depletion of O and OH is much smaller than the depletion on H. In other words, the heavy depletion of H is now compensated by moderate depletion of O and OH. Thus using O 2 in the feed gases increases the depth at which diamond could be deposited for a fixed CH 4/H 2 feed gas compositions. The deposition experiments were conducted at Lawrence Berkeley National Lab by Dr. Othon Monteiro. The predictions from our analysis and the experimental results match very well.
2.0 Diamond Homoepitaxial Studies
We are currently investigating the surface topographical evolution at nanoscale for defect formation & propagation during diamond homoepitaxy under the influence of intentional/unintentional impurities. The evolution of surface topgography is captured over a set of interrupted growth steps followed by detailed AFM (Atomic Force Microscopy) analysis. This is done by identifying certain features on the base diamond substrate and following the same feature over a series of growth steps. The evolution sequence of each feature gives us a comprehensive look at topographical evolution of the features that may lead to defect formations during growth.
For our studies we utilize well polished diamond substrates obtained from Sumitomo electric. We use tapping mode in Digital Nanoscope III for AFM scanning studies. Polishing diamond stones is very involved and can sometimes be more art than science. The polishing of diamond stones was possible due to our collaboration with Dr. James Butler from Naval Research Laboratory.




